The de Broglie relations
The de Broglie equations relate the wavelength and frequency
and frequency  to the momentum
to the momentum  and energy
and energy  , respectively, as
, respectively, as-
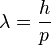 and
and 
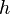 is Planck's constant. The two equations are also written as
is Planck's constant. The two equations are also written as is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),
is the reduced Planck's constant (also known as Dirac's constant, pronounced "h-bar"),  is the angular wavenumber, and
is the angular wavenumber, and  is the angular frequency.
is the angular frequency.Using results from special relativity, the equations can be written as
 is the particle's rest mass,
is the particle's rest mass,  is the particle's velocity,
is the particle's velocity,  is the Lorentz factor, and
is the Lorentz factor, and  is the speed of light in a vacuum.
is the speed of light in a vacuum.See the article on group velocity for detail on the argument and derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength).



0 comments:
Post a Comment